With its recognized expertise in special alloy processing and precision metallurgical part engineering for civil and military aeronautics, MICROSTEEL has successfully applied its know-how to the specific requirements of the medical device industry.
MICROSTEEL will deploy its high level of technical expertise and services to support your production of unfinished cast medical devices or with a more advanced machining and drag finishing service.
Study and production of implantable and non implantable medical devices

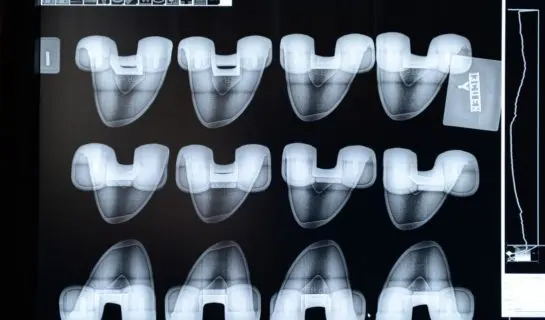
Development and manufacture of CoCr or stainless steel implantable and non implantable medical devices, compliant with your specifications and SOW: knees, semi-condyles, shoulders, cups, bases…
Production: a dedicated medical device manufacturing unit

MICROSTEEL manufactures your medical devices in a special unit dedicated to casting, heat treatment, machining, polishing, X-ray inspection and liquid penetrant testing.
Standard staples (CoCr ISO 5832-4) available in stock

Staple production outsourcing. Products delivered unfinished and sand blasted: responsibility for final cleaning, sterilization and CE marking lies with the client.
- Reinforced blount staples
- Reinforced oblique blount staples
- Staggered blount staples
- Straight serrated ligament staples
- Osteotomy staples
Certifications
MICROSTEEL’s industrial facilities are qualified for medical industrial processes:
- Compliance with EU Regulation 2017-745
- ISO 13485-2016 certification